|
|
CHEMICAL AND PHYSICAL CHANGESСтр 1 из 6Следующая ⇒ Передмова
Перед вами навчальний практикум, автори якого намагалися по-новому піднести старий матеріал. Відносна новизна викладу полягає в спробі здійснити формальну і змістовну реконструкцію матеріалу. Навчальний практикум призначено для вивчення англійської мови студентами І курсу технічних та механічних спеціальностей денної форми навчання. Практикум складено згідно з вимогами навчальної програми з іноземної мови і охоплює нормативну граматику англійської мови та базовий лексичний мінімум, дає можливість виробити та закріпити навички виконання завдань різного рівня складності. Мета практикуму – розвиток навичок розуміння й аналізу оригінальних текстів з основних проблем науково-технічного прогресу. Практикум складається із двох частин, у кожній - 8 розділів. Кожний розділ складається з трьох частин: лексичний, граматичний та додатковий. Додатково приведені професійно-спрямовані тексти та інформація, корисна для майбутніх фахівців технологів та інженерів.
Contents Unit 9..................................................................................................... 5 Unit 10..................................................................................................... 12 Unit 11.................................................................................................... 20 Unit 12.................................................................................................... 29 Unit 13..................................................................................................... 37 Unit 14.................................................................................................... 44 Unit 15.................................................................................................... 51 Unit 16.................................................................................................... 60 Additional Texts.................................................................................... 68 It is interesting to know......................................................................... 74 Unit 9
Text A Text B METALS
Metals are materials most widely used in industry because of their properties. The study of the production and properties of metals is known as metallurgy. The separation between the atoms in metals is small, so most metals are dense. The atoms are arranged regularly and can slide over each other. That is why metals are malleable (can be deformed and bent without fracture) and ductile (can be drawn into wire). Metals vary greatly in their properties. For example, lead is soft and can be bent by hand, while iron can only be worked by hammering at red heat. The regular arrangement of atoms in metals gives them a crystalline structure. Irregular crystals are called grains. The properties of the metals depend on the size, shape, orientation, and composition of these grains. In general, a metal with small grains will be harder and stronger than one with coarse grains. Heat treatment controls the nature of the grains and their size in the metal. Small amounts of other metals (less than per cent) are often added to a pure metal. This is called alloying (легирование) and it changes the grain structure and properties of metals. All metals can be formed by drawing, rolling, hammering and extrusion, but some require hot-working. Metals are subject to metal fatigue and to creep (the slow increase in length under stress) causing deformation and failure. Both effects are taken into account by engineers when designing, for example, airplanes, gas-turbines, and pressure vessels for high-temperature chemical processes. Metals can be worked using machine-tools. The ways of working a metal depend on its properties. Many metals can be melted and cast in moulds, but special conditions are required for metals that react with air.
Ex. 17. Answer the questions: 1. What are metals and what do we call metallurgy? 2. Why are most metals dense? 3. Why are metals malleable? 4. What is malleability? 5. What are grains? 6. What is alloying? 7. What is crystalline structure? 8. What do the properties of metals depend on? 9. What changes the size of grains in metals? 10.What are the main processes of metal forming? 11.How are metals worked? 12.What is creeping? Ex. 18. Find the following words and word combinations in text B: Властивості металів Відстань між атомами Правильне розташування Дуже різняться за своїми властивостями Кристалічна структура Розмір зерен Форма зерен Волочіння Прокатка Ковка Екструзія Структура і властивості зерна Гаряча обробка Втома металу Повзучість металу Плавка та відливання у форми Видиобробкиметалів Ex. 19. Complete the following sentences: 1. Metals are... 2. Metallurgy is... 3. Most metals are... 4. The regular arrangement of atoms in metals... 5. Irregular crystals... 6. The properties of the metals depend... 7. Metals with small grains will be... 8....controls the nature of the grains in the metal. 9. Alloying is... 10. All metals can be formed by... 11. Creep is... 12. Metals can be worked using...
Ex. 20. Explain in English the meaning of the following words: 1. malleability 2. crystalline structure 3. grains 4. heat treatment 5. alloying 6. creep Ex. 21. TranslateintoEnglish: 1. Метали – щільні матеріали тому, що у металах між атомами мала відстань. 2. Метали мають кристалічну структуру через правильне розташування атомів. 3. Чим менші зерна, тим твердіший метал. 4. Легування змінює структуру зерна і властивості металів. 5. Метал деформується і розширюється через втому та повзучість. Unit 10
Text A SUPERDENSE WATER
There is a tradition among physical chemists to regard the In contrast to crystalline solids liquids do not exhibit variability. These and some other characteristic properties ofliquids were the reason that the commonest of them water was adopted as the basis for the definition of several international standards of measurement. The choice of a cubic centimetre of water as the international unit of mass has been motivated by the fact that any given sample of water has a maximum density at a special temperature, near which small changes in the temperature cause very small changes in the density, and hence negligible changes in the mass of standard volume. Having carried out a number of investigations the scientists made a very interesting discovery, i.e. a new stable formof water having the density almost one and a half times that of ordinary water was obtained. This water was named Water II.Having examined its properties they found out that its index of refraction was equal to about 1.48 to 1.49. But when WaterII was diluted with ordinary water (Water I) this value dropped to the value for pure water, i.e. 1.33. Having continued their work, the scientists stated that the density of Water II was equal to that of pure water. Then it was veryimportant to learn the stability of the molecules of Water II and the forces responsible for their stability. It was assumed, that under the impact of the electrons on the molecules of Water II, these molecules are immediately broken down into ordinary molecules of H20. And that Water II in solution has a definite molecular weight that is approximately 10 times larger than that of ordinary water. Many scientists working in this field got interested in it. Thus, the scientist O. F. Vasiliev has expressed the hypothesis that noctilucent clouds consist of drops of Water II, which in contrast to drops of ordinary water may remain at a height of 90 kilometres without being evaporated. Some scientists suggested the possibility that on Venus water exists mainly in the form of Water 11. This question attracted the attention of many scientists and will be discussed in future. II Water is hydrogen oxide, a compound of hydrogen and oxygen. It can be made if hydrogen or a hydrogen-containing substance are burnt in air or oxygen. Most of the world's water is liquid, but an important fraction is solid as ice and snow. Many mineral substances contain water of crystallization (e.g. copper sulphate) and in the atmosphere there are millions of tons of water vapour. Clouds consist of minute droplets of water or crystals of ice. Water dissolves a very large number of substances and is the most important solvent. It does not dissolve greasy, fattysubstances or most plastics. Having found the composition of water, the scientistscould investigate its properties. It was stated that ordinary water is impure, it usually contains dissolved salts and dissolved gases, and sometimes organic matter. For chemical work water is to be purified by distillation.Pure water is colourless, tasteless and odourless. Rain water, formed by the condensation of water in the air, is nearly pure water, containing only small proportions of the dust and of dissolved gases. Having examined the properties of water, the chemists found that physical properties of water can be used to define many physical constants and units. The freezing point of water (saturated with air at 1 atm pressure) is taken as 0°C and the boiling point of water at 1atm is taken as 100°C. The unit of volume in the metric system is chosen so that 1ml of water at 3.98CC (the temperature of its maximum density) weighs 1.000 g/cm3. So, water is one of the most important of all chemical substances. It is a major constituent of living matter and of the environment in which we live.
Ex. 1. Answer the following questions: 1. How do physical chemists regard the properties of liquids? 2. What is the difference between liquids and gases? 3. What is the difference between liquids and crystalline solids? 4. Why was water adopted as the basis for the definitionof several international standards of measurement? 5. Whatis the choice of a cubic centimetre of water as the internationalunit of mass motivated by? 6. What is the index of refraction of water equal to? 7. When are the molecules of Water II broken down into ordinary molecules of H20? 8. What hypothesis has the Russian scientist O. F. Vasiliev expressed? 9. What is water? 10. What substances can't be dissolved in water? 11. What properties of water do you know? Ex.2. Find the pairs of synonyms and remember them: At once, learn, stable, mainly, several, chiefly, find out, almost, immediately, get, nearly, obtain, invariable, some Ex. 3. Find the pairs of antonyms and remember them: Impossible, stable, purity, solidify, unstable, indefinite, liquefy, impurity, definite, possible, seldom, odd, artificial, even, irregular, natural, regular, unstable, possible, stable, impossible, frequently.
Ex. 4. Open the brackets choosing a suitable word: 1. Isotopes occur more (seldom, frequently) in other elementsthan in hydrogen. 2. The chemical properties of isotopes are (different, identical) with those of regular atoms. 3. At present, about 1,000 (natural, artificial) radioactiveisotopes are known. 4. The (even, odd) atom has a neutron in the nucleus as well as a proton. 5. The use of new atomic power sources of (tremendous, small) energies is of great significance. 6. Using isotopes it is possible to make the control of pig-ironand steel production process (cheaper, more expensive). Ex. 5. Translate the following sentences paying attention to the words in bold type: 1. Rain water is nearly pure water. 2. Sea water contains nearly Ex. 6. Open the brackets choosing the correct forms of the Participles: 1. When (heating, having heated) this substance, one should be very careful. 2. (Investigated, having investigated, investigating) all the properties of new water, they could understand the mystery of silvery clouds. 3. The (dissolving, dissolved, having dissolved) materials may be soluble solids, liquids or gases. 4. Water (using, used, having used) in steam boilers, should be free from substances that cause corrosion. 5. (Purified, having purified, purifying) the water from the substance that cause corrosion, we can use it in steam boilers. Ex. 7. Translate the following into English: Порівнюючи їх з газами, які ми бачимо... Виконавши (провівши) ряд досліджень, науковці... Продовживши свою роботу... Дощова вода, утворена конденсацією водяної пари,... Провівши точне визначення складу води... Ex. 8. Read the text fluently and render it: Salts are insoluble in most solvents but they are soluble in water. Gasoline, benzene, carbon disulphide are good solventsfor grease, rubber, organic materials generally, but they do not dissolve salts. The reason that water is so effective for salts is that it has a very high dielectric constant and its molecules tend to combine with ions forming hydrated ions. Both of these properties aredue to the large electric dipole moment of the water molecule. Having studied these properties, we can understand that water molecule has a considerable amount of ionic character. Ex. 9. Read the text and retell it: Liquid water in thin layers is colourless, but in deep layersit has a bluish colour. Pure water is odourless and tasteless. Natural waters havetaste because of the presence of dissolved substances. Water is a poor conductor of electric current. Several common units of measurement are defined in terms of the properties of water. The melting point of ice and theboiling point of water at a pressure of one atmosphere were selected arbitrarily.
Grammar. Ex. 10. Form the Perfect Participle according to the models: Model I: totake – having taken (Active) to forget, to bring, to come, to carry out, to get, to find, to found, to translate, to compare. Model II: to take – having been taken (Passive) to solve, to receive, to build, to find, to learn. Ex. 11. Replace the subordinate clauses by the Perfect Participle according to the model: Model: When they had finished their work, they left the lab. Having finished their work, they left the lab. 1. When the composition of the substance had been determined, the scientists compared it with some other substances. 2. When they had investigated all the properties, they couldstate that these gases were harmful. 3. After they had separated nitrogen from other gases, they obtained it in nearly purecondition. 4. When he had found the needed solvent, he couldcontinue his experiment. Ex. 12. Give the equivalents of the following: Having compared these two substances,... 2. Whencomparing these substances... 3. When compared, thesesubstances... 4. Comparing these substances... 5. Having examined this element,... 6. If examined, this mineral... 7. Examining this liquid... 8. While examiningthis liquid... 9. The substance examined... Ex. 13. Change the sentences according to the given models: Model I: As my work is very difficult, he helps me. My work being very difficult, he helps me. 1. As the range of application of stable isotopes is very wide, the scientists are greatly interested in it. 2. As thismachine works well, we can use it at our plant. 3. Since the speed of light is extremely great, we cannot measure it by ordinary means. 4. As their lectures begin in the morning, they are free at five o'clock. Model II: When radioactivity had been discovered, science made great progress in atomic physics. Radioactivity having been discovered, science made great progress in atomic physics. 1. When all the properties of the element had been discovered, it was much easier to use it. 2. After the new computer had been built, they could calculate the acceleration of the particles. 3. When the solution had been evaporated, they began to examine the residue left. Ex. 14. Compare the following pairs of sentences and translate them: 1. This scientist being in our country got interested in oursystem of education. Everything being ready, we can start testing the motor. 2. Having carried out a series of analyses, he could make some interesting conclusions. The scientist having carried out his investigations in the laboratory, we could ask him about the results. 3. The phenomenon discovered by him helped us greatly in our research work. The melting point having been discovered, it was possible to continue our research work. 4. A solution containing no excess of either acid or basic hydroxide is known as a neutral solution. A solution containing no excess of an acid or basic hydroxide, we can call it a neutral one.
Ex. 15. Find the Absolute Participle Construction: A. 1. The room being dark, we couldn't see anything. 2. The book being translated into many languages, everybody will be able to read it. 3. Peter having passed his exams, we decided to have a rest in the country. 4. We went for a walk, our dog running in front of us. 5. The testwork having been written, he gave it to the teacher and left the room. 6. They having arrived at the station early, all of us went to the cafe. 7. My friends decided to go to the park, the weather being warm and sunny. 8. Our library buying all the new books, we needn't buy them ourselves. 9. The fuel burnt out, the engine stopped. 10. Many scientists worked in the field of mechanics before Newton, the most outstanding being Galileo. B. 1. Numerous experiments having been carried out at the orbital stations, it became possible to develop new methods of industrial production of new materials. 2. President Jefferson having offered his personal library, the foundation of the Library of Congress was laid. 3. Anthony Panizzi designed the Reading Room of the British Museum, the Reading Room being a perfect circle. 4. A beam of light being transmitted forwards, it is possible to measure the distance between the car and the other cars in front of it. 5. The distance having been measured, the computer adjusts the car's speed. 6. Two metallurgists produced a new superplastic metal, the new steel showing properties identical to Damascus steel. 7. The young physicist having discovered Newton's error, other scientists confirmed it. 8. The first TV sets having been shown in New York, the news about it spread throughout the world. С. 1. With the first steam engine built in the 17-th century, people began to use them in factories. 2. The inventor was demonstrating his new device, with the workers watching its operation attentively. 3. With his numerous experiments being over, Newton was able to write his work very quickly. 4. With the current being switched on, the machine automatically starts operating.
Ex.16. Translate the following sentences, mind the Absolute Participle Construction: A 1. The range of application of stable isotopes being very wide, the scientists are interested in them. 2. The electron is about as large as a nucleus, its diameter being about 10–12 cm. 3. Ordinary salt being examined with a magnifying glass, they saw that the crystals were of cubic form. 4. A gas can be dissolved in a liquid, the liquid changing its boiling point. 5. The elements having been arranged in the Periodic Table, it became easier to predict new elements. 6. The experiment being time-consuming, he has to spend much time in the laboratory. 7. Simple substances consist of atoms, each substance having its own special atom. B 1. The experiment having been over, the professor left the laboratory. 2. The professor spoke on the development of new technology, the lecture being illustrated by diagrams. 3. The work having been completed, we went home. 4. It being very cold, I made up my mind not to go out. 5. The translation of the article having been finished, I showed it to the teacher. 6. The professor having finished his lecture, we began to discuss it. 7. The weather being fine, we went out for a walk. 8. The difficulties having been overcome, we went on making further experiments. 9. The book being interesting, we read it with pleasure. 10. Our friends having helped us, we proceeded with our work. 11. There being no trams at that hour, we had to walk. 12. The professor walked into the lecture hall, the students following him. 13. The dictionaries having been brought, the students started translating articles from the new magazines. 14. Electrons moving through a wire, electrical energy is generated. 15. The current flow having been changed, the direction of the magnetic lines of force changed as well. 16. Water exists as ice at low temperatures and as steam at higher temperatures, with the temperature depending upon pressure. C 1. The experiment being very interesting, we work readily. 2. The range of application of stable isotopes being very wide, the scientists are interested in them. 3. The electron is about as large as a nucleus, its diameter being about 10-12cm. 4. Ordinary salt being examined with a magnifying glass, they saw that the crystals were of cubic form. 5. A gas can be dissolved in a liquid, the liquid changing its boiling point. 6. The elements having been arranged in the Periodic Table, it became easier to predict new elements. 7. The experiment being time-consuming, he has to spend much time in the laboratory. 8. Simple substances consist of atoms, each substance having its own special atom. Text B Unit 11
Text A
LIQUIDS
The liquid state occupies an intermediate position between the gaseous and solid states, liquid having a definite volume but no definite shape. Like a gas, a liquid can take the shape of any vessel in which it is put, but in contrast to a gas, a definite quantity of liquid is required for filling the vessel. A liquid can't be compressed so much as a gas because its molecules are already close together, large pressure producing small changes in volume. Increasing in temperature increases the kinetic energy of all molecules. The change of a liquid into the gaseous or solid states being dependent upon the kinetic energy of the molecules, which in turn is dependent upon the temperature, there are definite temperature characteristics for most liquids at which these changes occur. They are known as transition temperatures. If we place one liquid layer carefully on top of a layer of a more dense liquid in which it is soluble, and set the vessel where it won't be disturbed, we shall see that two liquids begin gradually mixing. It is also to be taken into consideration that all liquids do not flow with the same ease, water, alcohol, gasoline flowing easily, while heavy oil, glycerin flowing very slowly. When a liquid flows, layers of molecules begin rubbing over each other, friction being generated by this rubbing of layers of particles. The greater the friction, the slower is the flow. A liquid, which resists flowing, or resists the action of any other deforming force upon it, results in a homogeneous solution. We give this example for illustrations that the molecules of a liquid diffuse, though much more slowly than do those of a gas. The molecules of a liquid are much closer together than they are in a gas, because of the greater relative strength of attraction, the density of liquids being much greater. Naturally as the volume of a liquid begins varying with temperature its density will also start varying with temperature. It should be noted that the closeness of the molecules also is known as viscous, the opposite of viscosity being fluidity. Viscosity diminishes and fluidity increases with temperature. Evaporation The molecules within the interior of a liquid have a definite average energy of motion, and thus a definite mean velocity at each temperature. Some of them, however, at any given instant have a velocity sufficiently greater than the average velocity and this enables them breaking through the surface layer of molecules and escaping. Escaping of molecules from a liquid into its vapour is called evaporation.
Ex.1. Answer the following questions: 1. What position does liquid state occupy? 2. What shape does liquid take? 3. Is it possible for a liquid to be compressed? 4. Why is it impossible? 5. What does the change of a liquid into the gaseous or solid states depend on? 6. What will happen if we place one liquid layer on top of a layer of a more dense liquid in which it is soluble? 7. Do all liquids flow with the same ease? 8. What is the friction generated by? 9. Why are the molecules of a liquid much closer together than
Ex. 2. Translate the following sentences paying attention to the meanings of the words in bold type: 1. The temperature of the sun is intensely high. 2. This substance is highly insoluble in water. 3. The increase of energy stored in the gas, because of the rise in temperature, is called the increase of internal energy. 4. Solids that conduct
Ex. 3. Translate the following sentences paying attention to the meanings of "any": 1. At any temperature the molecules of gases can have the same kinetic energy. 2. Any body when heated to a sufficient high temperature becomes a source of light. 3. There is hardly anybody who doesn't know this law. 4. In any chemical compound the algebraic sum of the valence charges, or numbers, must be zero. 5. Give us any book on the history of Ukrainian culture. 6. Have you got any new devices in your laboratory? 7. They couldn't get any articles dealing with the development of rubber industry. 8. Any student who is interested in research work can take part in the work of our scientific society. 9. Many of the metals occur more extensively in silicates than in any other compounds, but the silicates are not used so extensively as ores because of difficulties involved in the production of the metals. 10. The classification of a particular element as a metal is based on a consideration of all its properties rather than any single property. 11. Having entered the laboratory, I couldn't see anybody at first as it was very dark there. 12. This substance is to be purified before using, at any rate.
Ex. 4. Find the pairs of antonyms and remember them: Rapidly, liquid, definite, decrease, solid, indefinite, increase, ease, heavy, difficulty, light, slowly.
Ex. 5. Translate the sentences, mind the use of "for": 1. This new machine has been working for twelve hours without stopping. 2. This semiconductor was used for the first time at our plant. 3. The use of charcoal and other adsorbents for the removal of impurities from a substance in solution has long been common practice. 4. For this reason this material couldn't be used as a conductor. 5. There is one use for which the carbon lamp suits better than the tungsten lamp. 6. Photoelectric cells are known to be used for detecting flaws in certain products. 7. For centuries glass was used for jewelry, ornaments and mosaic.
Ex. 6. Remember the meaning of the following words: feature – риса, особливість future – майбутнє thorough – ретельний, грунтовний through – через low – низький law – закон
Ex. 7. Translate the following sentences according to the model: Model: The higher the temperature, the greater is the amount of heat evolved. 1. The stronger the acid, the greater is the tendency to lose protons. 2. The stronger the magnification, the greater is the possibility to detect whether the body is homogeneous. 3. The faster an object moves, the greater is the air resistance. 4. The lower the atomic weight or atomic number of the inert gas, the lower are its boiling and melting points. 5. The larger the diameter, the smaller is the resistance and hence, the more current will flow through it. 6. The greater the difference in temperature between two points, the more heat will flow per second. 7. The greater the number of free electrons in a substance, the better that substance is a conductor of electricity.
Ex. 8. Translate the following sentences: 1. It is this article that shows the progress of our industry. 2. It was D. I. Mendeleyev who first classified the elements according to their atomic weights. 3. It is the development of chemical processes that his report deals with. 4. It was not until 1911 that the first theory of atomic structure was suggested 5. It was the Dutch physicist, Christian Huygens, who offered an explanation for this new phenomenon. Ex. 9. Translate the following sentences: 1. Though these molecules are very small the examination under a very powerful electronic microscope may show that they do exist. 2. He did carry out this experiment without any outside help. 3. Water does dissolve some substances readily. 4. This metal does conduct electricity well. 5. Though this subject was very difficult for him he did master it well. 6. All of the weight relations in chemical reactions do depend upon the weights of the atoms of the elements. 7. The process of extraction does involve four steps. 8. These electrode reactions, like other chemical reactions, do occur in steps. Ido know this man very well.
GRAMMAR
The Gerund Study and remember the following chart:
Ex. 10. Change the following sentences as in the models: Model I: He will wash glassware before he leaves. He will wash glassware before leaving. 1. We shall discuss this plan before we begin our work. 2. Before I translate the article Ishall read it thoroughly. Model II: After school he began to attend high courses. After school he began attending high courses. 1. As he was tired he stopped to read the report and left for home. 2. They started to make experiments in the second year. 3. They finished to look through the papers as soon as they had found the article concerned. 4. As I am very busy I can't continue to attend these lectures. Model III: When he came into the laboratory he came up to me. (on) On coming into the laboratory he came up to me. 1. You can get better results if you repeat the analysis. (by) 2. He will be able to translate the article after he repeats all the words, (after) 3. When he leaves the office he usually locks the door, (on) 4. Excuse me that I came so late, (for) Ex. 11. Use the Gerund of the verbs in brackets: 1. On (to get) good results he will inform me. 2. He succeeded in (to find) the book he needed. 3. His work resulted in (to discover) new elements. Ex. 12. Translate the sentences, mind the Gerunds: 1. Solid bodies have the property of keeping their shape without supporting of a vessel. 2. Upon being heated, the molecules begin moving very quickly. 3. If two glass rods are charged by rubbing them with silk, it is possible to watch a very interesting phenomenon. 4. Without being treated thissubstance cannot be used. 5. At last our research-workers succeeded in getting good results. 6. The teacher insists on carrying out this experiment in our laboratory. 7. Upon carrying out a number of experiments our students solved many interesting problems. 8. By using this law we define a unit charge of electricity. 9. Heating the wire from 0° to 100° increases its resistance approximately by 40%. 10. This experiment shows the increase of reaction velocity with increasing temperature. Ex. 13. Replace the Subordinate Clauses by the Gerund according to the models: Model I: After the student carried out his experiment he cleaned the laboratory bench. After carrying out his experiment the student cleaned the laboratory bench.
Model II: When they had finished their experimental work, they could write the report. After having finished their experimental work, they could write the report. 1. When they passed their last examination, they came to me. 2. After they had purified the water they could use it for drinking. 3. Before he began the translation of that article he had read it twice.4. When he discovered that new phenomenon he told us about it at once. 5. Before he left he had prepared everything for us. Ex. 14. Translate the sentences, mind the Gerund and Gerundial Constructions: 1. One of the most common methods of dissolving precipitates is their chemical conversion into soluble products.2. Besides finding out the type of a given reaction a chemist wants to know what products of the reaction he'll receive. 3. After being recrystallized from ethyl alcohol the solid be gan melting. 4. In writing formulas, the symbol for the more positive element is written first, as in NaCl. 5. After having investigated this strange phenomenon they succeeded in solving this problem. 6. It is because of this reaction with water that sodium must be protected from the moisture of the air by being kept under kerosene. 7. You should stop speaking at the lectures. 8. We know of the new laboratory having been built in our Institute.
Ex. 15. Translate the following sentences, mind the "ing"-forms: 1. After melting, the average speed of the moleculesremains the same as before but the molecules are now free of each other. 2. The process of overcoming the attractive forces between the molecules of a substance is called melting. 3. The only difference between waves transmitting heat radiation and radio waves is the difference in the wave length. 4. Instead of increasing the temperature of the ice, the energy is used in decreasing the attraction between the ice molecules. 5. The temperature of the melted ice rising, the movement of its molecules is speeded up. 6. In the process of boiling heat is constantly added to the liquid. 7. When a liquid boils evaporation takes place throughout the volume of the liquid, small bubbles of vapour forming within the liquid. 8. By increasing the pressure, however, the substance can be obtained in a liquid state, provided the change from liquid to solid is accompanied by an expansion. 9. As we have seen, adding heat to a substance will not always cause a rise of its temperature. 10. It is only the fast moving molecules that are able to escape from a liquid surface. 11. When a liquid starts boiling at a certain temperature and under a given pressure, the heat causes the liquid to vaporize.12. Every liquid can have a definite vapour pressure, this pressure increasing with rising temperature. 13. The vapour pressure of a liquid becoming equal to atmospheric pressure, the liquid boils. 14. They succeeded in getting good resultsafter a number of tedious investigations. 15. The steam expanding its volume will increase. 16. This resulted in increasing' the temperature greatly. 17. The rate of evaporation also depends on evaporating surface. 18. Since gases expand on heating and contract on cooling, it is interesting toconsider, what will happen if we continue lowering their temperature. 19. A thermometer bulb exposed to direct sunlight becomes much hotter than the surrounding air. 20. Using a thermometer, it is possible to transform power at low voltage into power at high voltage, power at high voltage being also transformed into power at low voltage.
Ex. 16. Translate the following sentences, mind the words in bold type: 1. Having absorbed much heat, the aluminium when it is cooled can give up the same quantity of heat. 2. The energy lost by the hot water is equal to the energytransferred to the cold water. 3. The wire being very thin and the current being large, the amount of generated heat is greater than that in the thick wire. 4. The wire, being very thin, was very good for our experiment. 5. The melting point of pure iron is about 1,535°C, most steels melting at about 300 to 500°С. 6. A gas being heated at constant pressure, work is done by the gas when it expands. 7. The problem having been solved, we could go home.8. The vapour pressure of a solid at any temperature being greater than one atmosphere, the substance will pass directly from the solid to the vaporous condition. 9. A solid body having been melted, the change of state took place at a definite temperature. 10. Experiments using a constant source of heat show that heat must be supplied to melt the solid. 11.The quantity of heat required for the changing the unit mass of a substance from the solid to the liquid state without any change of temperature is called the latent heat. 12. The liquid, being lighter than mercury, rises. 13. The silicon atom, being only slightly heavier than the aluminium atom and a bit smaller in radius, is also very active. 14. Having four electrons in its outer level with light electrons in the next lower level, the atom may attain stability by either losing four lectrons or gaining four electrons. 15. The process of electrification is, therefore, not one of creating electricity but of transferring electrons from one material to another. 16. In passing through the metal electrons must collide with many ions. 17. In making up a condenser, one has to take into consideration its size, as the amount of charge that a condenser stores depends on its size.
Ex. 17. Translate the text using a dictionary. Analyse the "ing"-forms: A liquid being cooled, its molecules lose energy. The cooling process being conducted slowly, the particles which constitute the solid may arrange themselves into definite positions. The solid itself will exist in a regular geometric form called a crystal. Crystals are of various forms and of varying degrees of strength and rigidity, both these properties depending upon the substance of which they are composed. Having learned this, we can state that the properties of matter in the gaseous state are determined largely by the motion of particles, while in the solid state they are determined largely by the rigidity of the structure formed and by the position which the particles occupy with respect to each other in the structure, these latter being dependent upon the magnitude of the forces of attraction and upon the size of the particles.
Ex. 18. Translate the following text analysing the functions of the Gerund: A saturated solution of one of the numerous solids, which are more soluble at higher temperatures than at lower ones should, upon being cooled, deposit crystals of the solute until equilibrium is established at the lower temperature. If, however, the saturated solution at the higher temperature contains no undissolved solute and no particles of foreign matter, and if it is allowed to cool without being disturbed in anyway, it is often possible to cool it without crystallizing ofthe excess solute occurring. A solution which has been so cooled naturally contains more solute than can normally be dissolved in the given quantity of solvent at the existing temperature.
Text B Conductivity It will be interesting to note that an iron wire of the same length as a copper one has a greater resistance. Under the same conditions, the copper wire allows more current flowing than the iron wire. Copper has a greater conductivity. Conductivity means the ability of carrying the current. The unit of conductivity is the siemens or the mho. The unit of resistance is the Ohm. In 1826 Ohm found a simple correlation between resistance, current and voltage. He also observed that if the voltage remains the same, the greater the resistance, the smaller the current is. So, it can be stated: the current that flows in a circuit is directly proportioned to the voltage and inversely proportioned to the resistance. Ex. 24. Translate the following text: The Metallic Elements About seventy-nine of the one hundred substances are metals. A metal may be defined as a substance, which has large conductivity of electricity and of heat, has a characteristic luster, called metallic luster, and some other properties. In addition, the electric conductivity increases with decrease in temperature. The metals themselves and their alloys are of great usefulness to man. The importance of some alloys is due primarily to their hardness and strength. These properties are a consequence of the presence in the metals of very strong bonds between the atoms. For this reason, it is of great interest to us to understand the nature of the forces holding the metal atoms together in these metals and alloys. First, we should consider an alloy to be a metallic material containing two or more elements. It may be homogeneous, consisting of a single phase, or heterogeneous, being a mixture of phases.
Unit 12 Text A: Air Text B: Dies Grammar: The Conditional Clauses
Words to remember:
Text A AIR Liquid Air Liquid air is a mixture of the liquefied gases. It is a milky liquid owing to the presence of solid carbon dioxide and ice. If these solids were removed by filtering, the filtrate would have a pale blue tint. Liquid air due to its extremely low temperature produces remarkable physical changes. If a tin or iron vessel were cooled by liquid air, it would become so brittle that it could be crushed with the fingers.Mercury freezes so hard in liquid air that it could be used as a hammer. It could be hardly crushed. If the liquid air were in a tea kettle standing on a block oi ice, the liquid air would boil vigorously. If the kettle of liquid air were placed over a lighted Bunsen burner, frost and ice would collect on the bottom of the kettle. Ordinary liquid air is from one half to one fifth liquid oxygen, and therefore it can support combustion. A red hot rod of steel would have burned brilliantly if it had been placed in the liquid air. Lately numerous applications of liquid air have been proposed and have been used in all branches of life. For example, it can be used to remove diseased flesh from the wounds. It can be widely used as commercial source of oxygen and nitrogen. Liquid air can be readily manufactured in large quantities at a comparatively low cost, that is why liquid air is made use of both in industry and in everyday life. Ordinary Air Ordinary air contains nearly constant proportions of three elementary substances, viz. nitrogen – 75.4 per cent, oxygen – 23.2 per cent and argon – 1.2 per cent by weight. It also contains small proportions of several inactive gases in some proportions of carbon dioxide, water vapour and lust. The relative proportion of water vapour in the air could be stated in terms of relative humidity, i. e. the ratio between the concentration of water vapour in the air and the concentration required for equilibrium at the same temperature. If the relative humidity were low, evaporation would occur rapidly. If the relative humidity were high, evaporation would occur slowly as the air is nearly saturated.
Ex. 1. Answer the following questions:
1. What is liquid air? 2. What are the properties of liquid air? 3. What changes can liquid air produce? 4. When does an iron vessel become brittle? 5. What freezes hard in liquid air? 6. What do you know about ordinary liquid air? 7. Where is liquid air applied? 8. What does ordinary air contain? 9. When can evaporation occur rapidly? 10. What would happen if the relative humidity were high? Ex. 2. Find the right statement: I. Liquid air can be manufactured.... a) in small quantities at a high cost; b) in large quantities at a high cost; c) in small quantities at a comparatively high cost; d) in large quantities at a comparatively low cost. II. Liquid air is.... a) a mixture of solid gases; b) a compound; c) an element; d) a mixture of the liquefied gases.
Ex. 3. Find the pairs of synonyms and remember them:
To use, to produce, due to, to manufacture, quickly, to employ, rapidly, owing to. Ex. 4. Find the pairs of antonyms and remember them:
Active, to solidify, warm, solid, inactive, the same, cool, to liquefy, different, liquid. Ex. 5. Choose the equivalents from the right column:
both... and також, як nearly обидва near майже as well as завдяки due to як..., так і both коло, біля
Ex. 6. Translate the following sentences mind the words in bold: 1. In terms of the given formula N40 we can say that air is a mixture of nitrogen and oxygen. 2. Water is sometimes cloudy due to the presence of some particles. 3. They can hardly solve the problem without you. 4. We know that ice can freeze hard if the temperature is low. 5. The lecture was attended by nearly all the students. 6. If we work hard, we shall certainly finish our work soon. 7. Air conditioning requires the regulation of both the temperature and humidity. 8. When the air is not in circulation, the layer near the body becomes nearly saturated. 9. The transition point at which a change takes place is described in terms of temperature and pressure. Ex. 7. Translate the following sentences, state the function of the words in bold type:
1. Both copper and tin melt at a low temperature. 2. The reaction of acids with metal hydroxides is a completed reaction because of the low degree of ionization of water. 3. The only manganic compounds which may be prepared in aqueous solution are those of low solubility. 4. If a highly compressed gas composed of molecules is allowed to expand quickly to low pressure, its molecules overcome the forces of attraction, consequently, the temperature of the gas is lowered. 5. Ceramic products could be characterized by low thermal conductivity. 6. The freezing point lowers in this reaction. 7. The scientist tried to lower the concentration of the absorbed substance in the reaction. Ex. 8. Find the sentence where the word “change” is a verb. Translate the following sentences: 1. This is well known that changes take place in all the substances. 2. Chemical changes are usually permanent. 2. Chemical changes are usually permanent. 3.The changes that take place during the freezing of a liquid are opposite to those that take place during the melting of the solid. 4. There is little change in the concentration of complex anions so long as the solution is acidic. 5. It has been shown that every chemical change could be accompanied by a definite energy change. 6. The vapour pressure of a liquid is affected by changes in temperature. 7. If you changed the temperature, the reaction would go to completion. Ex. 9. Open the brackets translating words into English: 1. (На основі) the kinetic theory, the energy of molecules increases with rise in temperature. 2. When he was a student he (бувало) spend a lot of time in the reading-room of this Institute. 3. Oxygen is also (використовується) to maintain air composition for respiration at high altitudes. 4. I shall finish my work (яктільки) I get good results. 5. They can (ледве) finish their research without his help. 6. (Останнійчас) many new articles dealing with this subject have been published. 7. The heat of combustion of acetylene, (тобто) the number of calories evolved per mole, is large. 8. The most extensive industrial (використання) of oxygen is in the production of high temperatures in oxygen-gas flames. 9. Ozone decomposes slowly at room temperature but rapidly and (легко) at 250°. 10. The reactions of ozone are of the same variety (как) those of oxygen. 11. This (термін) can be (використаний) by us. 12. (Ще) in the 16th century the scientists knew this reaction. 13. (Зміна) in temperature results in the (зміни) of solubility. 14. The velocity of a reaction is (змінюється) by (змінюванням) in conditions. 15. The ventilation of a room requires (як) a sufficient supply of fresh air (такі) the circulation of the air. 16. Air conditioning requires the regulation of (як) the temperature (такі) the humidity.
Conditionals 1. I shall speak to him if I see him. (1) 2. I should speak to him if I saw him. (2) 3. I should have spoken to him if I had seen him. (3) 4. If the book were interesting, he would read it. 5. Were the book interesting, he would read it. 6. Provided he had this book, he would read it. 7. Had he this book, he would read it. Ex. 10. Analyse the Condition Clauses, translate them:
1. If there were no dust, the air would become supersaturated with water. 2. If air is cooled sufficiently, it can be converted into a liquid. 3. If the liquid air were allowed to evaporate, different components would predominate in the fractions obtained at different temperatures. 4. If the atmosphere continued to be of uniform density, we should find that it is 5 miles deep. 5. Were a quantity of silver chloride placed in a quantity of water, silver and chloride ions would begin to leave the surface. 6. Had water been added to the mixture, more alcohol and acid would have been formed. 7. Had not chemistry made possible the production of fundamental materials, radio and TV would have been unknown. Ex. 11. Change the sentences according to the model:
Model: The experiment is not interesting. I shall not carry it out. If the experiment were interesting, I should carry it out. 1. This article does not deal with organic chemistry. I shall not translate it. Ex. 12. Complete the following sentences according to the models: Model I: If I knew him,... (I should speak to him). 1. If she were in the laboratory, she... 2. Provided they prepared for their examination better, they... 3. Were he free, he... 4. Unless he came in time, he... Model II: If I had known him before,... (1 should have spoken to him). 1. If he had read that book, he... 2. If she had not been ill, she... 3. Provided they had come earlier, they... 4. If he had been in her place, he... Ex. 13. Open the brackets, use the correct verb forms: 1. If liquid boiled, nitrogen (to escape) from the solution more rapidly than oxygen, as its boiling point is lower than that of oxygen. 2. Provided a liquid had evaporated into a closed space, its gaseous molecules (to leave) the liquid surface. 3. Unless he helps me, I (to be able) to finish this work in time. 4. If we did not know the nature of radioactive elements, it (to be difficult) to deal with them. 5. If they had studied the activity of uranium, they (to understand) that phenomenon better. 6. If this molecule (to be) decomposed, we should obtain atoms. 7. Provided zinc were heated with sulphuric acid, the metal (to replace) hydrogen. 8. If someone weighed, say 80 kilograms at the North Pole, he (to weigh) less at the equator. 9. If sulphur burns in air or oxygen, the main product (to be) sulphur dioxide. Ex. 14. Supply the appropriate forms of the verb instead of the Infinitives in brackets: 1. The reaction will not take place, unless they (to add) a catalyst. 2. Provided you wanted to obtain pure nitrogen, you (to pass) gaseous ammonia over heated copper oxide. 3. If at ordinary temperatures molecules of a gas exhibit no attraction, Joule-Thompson effect (to be) not effective.4. If a liquid had been cooled gradually, it (to reach) a temperature at which crystals of the solid form (to begin), to appear. 5. Were this laboratory equipped well, it (to be) much easier to work in it. 6. Were the oxygen in the air reduced below a certain percentage, it (not to support) combustion. 7. Unless iron is heated red-hot, it (not to absorb) any carbon. 8. If you took a little salt and examined the grains, you (to see) that most of them are perfect cubes. 9. Provided there were no air, the piece of paper and the piece of iron (to fall) together. 10. If one or more of the reacting substa   ЧТО ПРОИСХОДИТ, КОГДА МЫ ССОРИМСЯ Не понимая различий, существующих между мужчинами и женщинами, очень легко довести дело до ссоры... 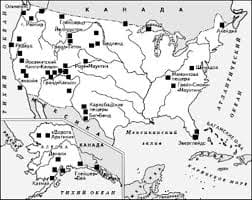 Система охраняемых территорий в США Изучение особо охраняемых природных территорий(ООПТ) США представляет особый интерес по многим причинам... 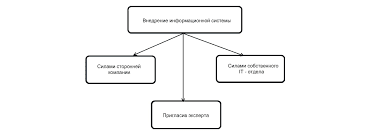 Что делает отдел по эксплуатации и сопровождению ИС? Отвечает за сохранность данных (расписания копирования, копирование и пр.)... 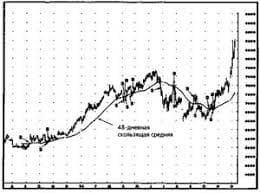 Что вызывает тренды на фондовых и товарных рынках Объяснение теории грузового поезда Первые 17 лет моих рыночных исследований сводились к попыткам вычислить, когда этот... Не нашли то, что искали? Воспользуйтесь поиском гугл на сайте:
|