|
|
Classification of lead acid batteries.Стр 1 из 2Следующая ⇒ Classification of lead acid batteries. lead acid batteries it is a type of most common batteries in our days. Main areas of application are: accumulator batteries for transports, emergency electric sources, backup power sources. Operating principle In the discharged state both the positive and negative plates become lead(II) sulfate (PbSO4), and the electrolyte loses much of its dissolved sulfuric acid and becomes primarily water. The discharge process is driven by the conduction of electrons from the negative plate back into the cell at the positive plate in the external circuit. Negative plate reaction Pb(s) + HSO−4(aq) → PbSO4(s) + H+(aq) + 2e Positive plate reaction PbO2(s) + HSO−4(aq) + 3H+(aq) + 2e− → PbSO4(s) + 2H2O(l) The total reaction can be written as Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l) Rated voltage produced by such a battery is 2 volts. Single batteries are connected in series to obtain higher voltage values. For example: a 12-volt battery consists of series-connected in a common housing, the six batteries. By construction, lead-acid batteries are maintenance-free and serviced. Serviced during operation requires some care (control level and electrolyte density). Maintenance-free - are airtight, operate in any position and does not require maintenance. There are the following basic types of lead-acid batteries, which can be used in stand-alone power supply systems: starter - you know them well, require maintenance, and ventilation. They have high self-discharge. AGM - these sealed, maintenance-free batteries do not require a ventilation space for the installation. Cheap AGM type batteries work well in buffered mode, ie, in the charging mode. This mode serves to 10-15 years. General purpose AGM batteries typically used in inexpensive ninterruptible Power Supply (UPS); UPS generally intended to crash, not to supply the load continuously. Therefore, given that they are usually in the offices there and put AGM batteries. Gel batteries more resistant to cyclic charge-discharge modes. Also, they seem to tolerate extreme cold. Reduced capacity with decreasing battery temperature is also less than other types of batteries. Gel batteries AGM batteries a bit more expensive and the more starter. Gel batteries are also different on purpose - there are both general-purpose and deep discharge. traction - Designed for cycling. More suited for stand-alone power supply systems (such as onboard). But much more expensive starter and gel AB normally have a liquid electrolyte, maintenance-free and ventilated installation room. There are special " solar " - specially designed for the "hard" cycling. It is best to work with the flood pasted battery plates (series OPzS). These batteries are specially designed for use in stand-alone power systems.
Draw a scheme of the cylindrical lithium-ion battery. A lithium-ion battery or Li-ion battery is a type of rechargeable battery in which lithium ions move from the negative electrode to the positive electrode during discharge and back when charging. Li-ion batteries use an intercalated lithium compound as one electrode material, compared to the metallic lithium used in a non-rechargeable lithium battery. The electrolyte, which allows for ionic movement, and the two electrodes are the constituent components of a lithium-ion battery cell. They are one of the most popular types of rechargeable batteries for portable electronics, with a high energy density, tiny memory effect and low self-discharge.
Description of fuel cells. The chemical current source in which electrical power is generated by chemical reaction between the reducing agent and oxidizing agent, continuously and separately supplied to the fuel cell electrodes from outside. The reaction products are continuously output from the fuel cell Anode reaction: H2 - 2e → 2H + (1) Cathodic reaction: ½ O2 + 2H + + 2e → H2O (2) Current-producing reaction: H2 + ½ O2 → H2O (3) The first fuel cells were invented in 1838. There are many types of fuel cells, but they all consist of an anode, a cathode, and an electrolyte that allows positively charged hydrogen ions (or protons) to move between the two sides of the fuel cell. The anode and cathode contain catalysts that cause the fuel to undergo oxidation reactions that generate positively charged hydrogen ions and electrons. The hydrogen ions are drawn through the electrolyte after the reaction. At the same time, electrons are drawn from the anode to the cathode through an external circuit, producing direct current electricity. At the cathode, hydrogen ions, electrons, and oxygen react to form water. As the main difference among fuel cell types is the electrolyte, fuel cells are classified by the type of electrolyte they use and by the difference in startup time ranging from 1 second for proton exchange membrane fuel cells (PEM fuel cells, or PEMFC) to 10 minutes for solid oxide fuel cells (SOFC). In addition to electricity, fuel cells produce water, heat and, depending on the fuel source, very small amounts of nitrogen dioxide and other emissions. The energy efficiency of a fuel cell is generally between 40-60% Biofuel element · Principle - the use of natural catalysts · Enzymes dehydrogenase responsible for oxidation and the formation of hydrogen, are unique effective non-platinum catalysts for these processes · Disadvantages: short service life and low power
Classification of lead acid batteries. lead acid batteries it is a type of most common batteries in our days. Main areas of application are: accumulator batteries for transports, emergency electric sources, backup power sources. Operating principle In the discharged state both the positive and negative plates become lead(II) sulfate (PbSO4), and the electrolyte loses much of its dissolved sulfuric acid and becomes primarily water. The discharge process is driven by the conduction of electrons from the negative plate back into the cell at the positive plate in the external circuit. Negative plate reaction Pb(s) + HSO−4(aq) → PbSO4(s) + H+(aq) + 2e Positive plate reaction PbO2(s) + HSO−4(aq) + 3H+(aq) + 2e− → PbSO4(s) + 2H2O(l) The total reaction can be written as Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l) Rated voltage produced by such a battery is 2 volts. Single batteries are connected in series to obtain higher voltage values. For example: a 12-volt battery consists of series-connected in a common housing, the six batteries. By construction, lead-acid batteries are maintenance-free and serviced. Serviced during operation requires some care (control level and electrolyte density). Maintenance-free - are airtight, operate in any position and does not require maintenance. There are the following basic types of lead-acid batteries, which can be used in stand-alone power supply systems: starter - you know them well, require maintenance, and ventilation. They have high self-discharge. AGM - these sealed, maintenance-free batteries do not require a ventilation space for the installation. Cheap AGM type batteries work well in buffered mode, ie, in the charging mode. This mode serves to 10-15 years. General purpose AGM batteries typically used in inexpensive ninterruptible Power Supply (UPS); UPS generally intended to crash, not to supply the load continuously. Therefore, given that they are usually in the offices there and put AGM batteries. Gel batteries more resistant to cyclic charge-discharge modes. Also, they seem to tolerate extreme cold. Reduced capacity with decreasing battery temperature is also less than other types of batteries. Gel batteries AGM batteries a bit more expensive and the more starter. Gel batteries are also different on purpose - there are both general-purpose and deep discharge. traction - Designed for cycling. More suited for stand-alone power supply systems (such as onboard). But much more expensive starter and gel AB normally have a liquid electrolyte, maintenance-free and ventilated installation room. There are special " solar " - specially designed for the "hard" cycling. It is best to work with the flood pasted battery plates (series OPzS). These batteries are specially designed for use in stand-alone power systems.
  Что делать, если нет взаимности? А теперь спустимся с небес на землю. Приземлились? Продолжаем разговор... 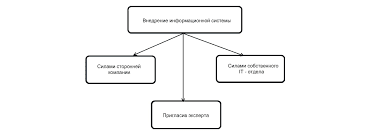 Что делает отдел по эксплуатации и сопровождению ИС? Отвечает за сохранность данных (расписания копирования, копирование и пр.)...  ЧТО И КАК ПИСАЛИ О МОДЕ В ЖУРНАЛАХ НАЧАЛА XX ВЕКА Первый номер журнала «Аполлон» за 1909 г. начинался, по сути, с программного заявления редакции журнала... 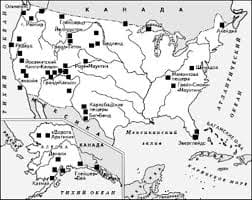 Система охраняемых территорий в США Изучение особо охраняемых природных территорий(ООПТ) США представляет особый интерес по многим причинам... Не нашли то, что искали? Воспользуйтесь поиском гугл на сайте:
|