|
|
You are using sulphate electrolyte for nickel electroplating. The formed coating peels from the substrate. What do you can propose for this problem solution? ⇐ ПредыдущаяСтр 5 из 5 Peeling of nickel plating is the most common form of rejection during nickel plating, especially with multilayer coating. The cause of exfoliation is diverse and it is not always possible to clarify them. Peeling of nickel coating is most often with poor pre-cleaning of the surface and insufficiently performed degreasing and pickling. Peeling of the nickel layer as a result of poor adhesion to the base metal can be observed after a considerable time after nickel plating. A break in the supply of current or a sudden change in the current density during the coating process can also cause nickel peeling. Peeling of nickel in the form of small shiny flakes, falling off from contact, can be at a low pH, i.e. increased acidity, with an overestimated current density, with electrolyte contamination with lead and chromium salts. A low concentration of nickel salts with a high content of conductive salts can also be a cause of delamination. Increased brittleness and cracking of the nickel coating can be observed in the presence of iron salts and impurities in the electrolyte with organic impurities. Often, in multilayered coatings, the insufficient thickness of the first copper layer from the cyanide bath or the nickel sub-layer and their porosity lead to further peeling of the subsequent coatings, because during copper plating in the acid electrolyte, a partially contact deposition of a loose layer of copper with poor adhesion to the base metal can occur. 31. The nickel anodes containing 90% Ni and 10% other impurities (cobalt, copper, iron) was prepared for refining. The anodic current efficiency (yield) of the nickel dissolution was 92.86% at 9000 A. Calculate the quantity of nickel dissolved from the anode during 1 hour. Ni I=9000A τ=60 min=1 h M(Ni)=58,7g\mol Ƞ=92,86% F=26,8 A• h m=? m=I• q• τ q=M/nF q=58,7/2*26,8 =1,01 g/A• h m=9000A • 1,01 g/A*h • 1 h= 9090 g =9,09 kg 9,09 – 100% X – 92,86% X = 9,09 • 100 / 92,86 = 8,44 kg m(Ni) = 8,44 • 90%/100%=7,596 kg Answer: m(Ni) = 7,596 kg 32. Chromium plating bath works at the cathodic current density j = 40 A/dm2, the current efficiency - 30% chromium. The required thickness of the chrome layer is 5 microns. Calculate the time of chromium plating. Сr j=40 A/dm2 Ƞ=30% d=5 micr=0.00005dm M(Сr)=52g\mol F=26,8 A• h ρ(Cr)= 7 190 kg/m3 τ=? d= j • q • Ƞ • τ / ρ → τ= d • ρ / j • q • Ƞ q=M/nF q=52g /3•26,8 A• h = 0.65 g/A• h τ= 0.00005dm • 7 190 g/dm3 / 40 A/dm2 • 0,65 g/A• h • 30 = 0.000461 h = 2 sec Answer: 2 sec Calculate the current efficiency if 0.48 g of copper was deposited at current 2.5 A during 10 minutes of the electrolysis of copper sulphate solution. Cu m=0,48 g I=2.5 A τ=10 min=0,166 h M(Cu)=63,5g\mol F=26,8 A• h Ƞ=? Ƞ = 100% • m(pract)/m(theor) m(theor)=I• q• τ q=M/nF q=63,5g /2•26,8 A• h = 1.18 g/A• h m(theor)= 2.5 A • 1.18 g/A• h • 0,166 h= 0.4897g Ƞ = 100% •0,48 g /0.4897g = 98% Answer: Ƞ = 98% Compute the practical current efficiency for chromium, if 7.19 g of chromium was deposited at current 40 A during 4 hours 16 minutes and 36 seconds. Cr m=7,19 g I=40 A τ=4 hours 16 minutes and 36 seconds = 4,23 h M(Сr)=52g\mol F=26,8 A• h Ƞ=? Ƞ = 100% • m(pract)/m(theor) m(theor)=I• q• τ q=M/nF q=52g /3•26,8 A• h = 0.65 g/A• h m(theor)= 40 A • 0.65 g/A• h • 4,23 h= 109,4 g Ƞ = 100% •7,19 g /109,4g = 6,57% Answer: Ƞ = 6,57%
35. The process of electrolytic nickel plating is carry out in a stationary bath at cathode current density = 4.0 A/dm2 with a current efficiency (yield) - 96%. The required layer thickness of nickel coating is 25 microns. Calculate the duration of the nickel plating process. Ni j=4.0 A/dm2 Ƞ=96% d=25 micr=0.00025dm M(Ni)=58,7g\mol F=26,8 A• h ρ(Ni)=8800kg/m3 τ=? d= j • q • Ƞ • τ / ρ → τ= d • ρ / j • q • Ƞ q=M/nF q=58,7/2*26,8 =1,01 g/A• h τ= 0.00025dm • 8800g/dm3 / 4.0 A/dm2 • 1,01 g/A• h • 96 = 0,0057 h = 20,4 sec Answer: 20,4 sec 36. Calculate the thickness of the zinc coating, which was obtained at the next conditions: time of the process = 20 min, surface S = 0,80 dm2, current density j = 3,2 A/dm2 and the cathode current efficiency = 84.4% for zinc. Zn j=3.2 A/dm2 Ƞ=84,4% M(Zn)=65\mol F=26,8 A• h ρ(Zn)= 7 133 kg/m3 τ=20 min = 0,33 h d=?1 d= j • q • Ƞ • τ / ρ q=M/nF q=65/2*26,8 =1,21 g/A• h d=3.2 A/dm2• 1,21 g/A• h • 84,4 • 0,33 h / 7 133 g/dm3=0.01512 dm = 1,512 mm Answer: d = 1,512 mm 37. Calculate the thickness of the nickel precipitate formed within 20 minutes, if the current efficiency is 90%, the current density is 3A/dm2. The density of nickel is 8902 kg/m3. Ni j=3.0 A/dm2 Ƞ=90% M(Ni)=58,7g\mol F=26,8 A• h ρ(Ni)= 8902 kg/m3 τ=20 min = 0,33 h d=? d= j • q • Ƞ • τ / ρ q=M/nF q=58,7/2*26,8 =1,01 g/A• h d=3.0 A/dm2• 1,01 g/A• h • 90 • 0,33 h / 8902 g/dm3=0,0101 dm = 1,01 mm Answer: d = 1,01 mm 38. Calculate the thickness of the gold coating on the wedding ring, which was obtained at the next conditions: time of the process = 10 min, current density j = 1,2 A/dm2 and the cathode current efficiency = 95%. Ring has internal diametr: 1sm 8 mm, external diametr: 1sm 10 mm, width: 1sm Au j=1.2 A/dm2 Ƞ=95% M(Ni)=197 g\mol F=26,8 A• h ρ(Au)= 19 300 kg/m3 τ=10 min = 0,166 h d=? d= j • q • Ƞ • τ / ρ q=M/nF q=197/1*26,8 =7,35 g/A• h d=1.2 A/dm2• 7,35 g/A• h • 95 • 0,166 h / 19300 g/dm3= 0,00721 dm = 0,73 mm Answer: d = 0,73 mm On the figure you can see working drawing of detail, which is necessary to cover by zinc. Calculate the current strength for this process.
Zn j=10 A/dm2 R=6,25 mm A=120 mm B=35 mm C=8 mm M=5 mm N=7 mm I=? I=j•S S=S(full)+S(Little Paral) – 4 S(circle) – 2S (Edge) S(full)=2(AB+AC+BC) S(Little Paral) = (AM+MN+AN) S(circle)= 2π R2 S (Edge)=AM S(full)=2(120*35+120*8+8*35)=10 880 mm2 S(Little Paral)=2(120*5+5*7+120*7)=1475 mm2 S(circle)= 2•3,14•6,252= 245,3125 mm2 S (Edge)=120*5= 600 mm2 S= 10 880 mm2 +1475 mm2 – 4*245,3125 mm2 -600 mm2 = 10 773,75 = 1,077 dm2 I=10 A/dm2 • 1,077 dm2 = 10,77 А Answer: I=10,77 А   Система охраняемых территорий в США Изучение особо охраняемых природных территорий(ООПТ) США представляет особый интерес по многим причинам...  ЧТО ПРОИСХОДИТ, КОГДА МЫ ССОРИМСЯ Не понимая различий, существующих между мужчинами и женщинами, очень легко довести дело до ссоры...  ЧТО ТАКОЕ УВЕРЕННОЕ ПОВЕДЕНИЕ В МЕЖЛИЧНОСТНЫХ ОТНОШЕНИЯХ? Исторически существует три основных модели различий, существующих между... 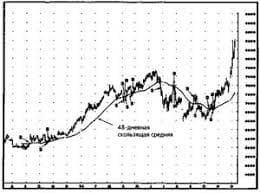 Что вызывает тренды на фондовых и товарных рынках Объяснение теории грузового поезда Первые 17 лет моих рыночных исследований сводились к попыткам вычислить, когда этот... Не нашли то, что искали? Воспользуйтесь поиском гугл на сайте:
|